CO2 emissions have caused a series of environmental problems, so the technology of converting CO2 into high-value products has been widely concerned by researchers. Natural gas (methane) accounts for a large proportion of the world's energy consumption. The generation of methane from CO2 by the hydrogenation method is a promising technology. However, there is still a big challenge to develop nickel-based methanation catalysts with good activity and stability at low temperatures.
Recently, a series of progress has been made in the cooperative researches on CO2 methanation between Dr. Peng Zheng (Key Laboratory on Resources Chemicals and Materials of Ministry of Education, Shenyang University of Chemical Technology) and Prof. Fabing Su (Institute of Process Engineering, Chinese Academy of Sciences):
(1) The reverse phase CeO2-Cr2O3 catalyst with controllable CeO2-Ni and Cr2O3-Ni interface structures was synthesized by ion exchange reverse loading method, and the effect of the interface between oxide and metal on the CO2 methanation performance was studied. Combined with Dr. Peng Zheng's theoretical calculation, it was revealed that the CeO2-Ni interface followed the *COOH reaction path, while the Cr2O3-Ni interface followed the CO path with a lower reaction barrier (Fig. 1). This work has been published in Applied Catalysis B: Environmental (IF = 22.1) (2023, 339: 123121. https://doi.org/10.1016/j.apcatb.2023.123121).
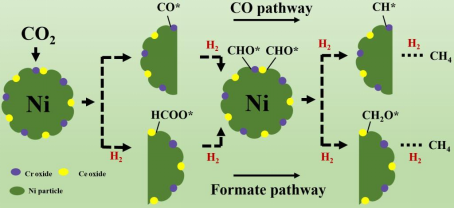
Fig 1. Schematic diagram of catalyst structure and reaction mechanism
(2) A series catalyst with double active centers composed of Ru single atom and Ni nanoparticles was prepared by hydrothermal and impregnation method (Fig. 2). The sites of CO2 adsorption and activation were studied. Combined with Dr. Peng Zheng's theoretical calculation, it was revealed that Ru1 site was more effective in converting CO2 to CO, while Ni site was mainly used to hydrogenation CO to CH4. This work has been published in Applied Catalysis B: Environmental (IF = 22.1) (2023, 323: 122190. https://doi.org/10.1016/j.apcatb.2022.12219).
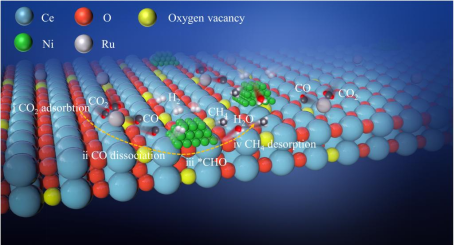
Fig 2. Schematic diagram of catalyst structure and reaction mechanism
(3) High temperature carbonization pyrolysis method was used to prepare NiO@Ni/NC catalyst. And the CO2 methanation with H2O as hydrogen source by photothermal synergistic catalysis was studied (Fig. 3). Combined with Dr. Peng Zheng's DFT calculation, it was revealed that H2O was easier to dissociate on the surface of NiO and form H2 on the surface of Ni, and *COOH was the key intermediate product of the reaction. This work has been published in Nano Research (IF = 9.9) (in press. https://doi.org/10.1007/s12274-023-6226-5).
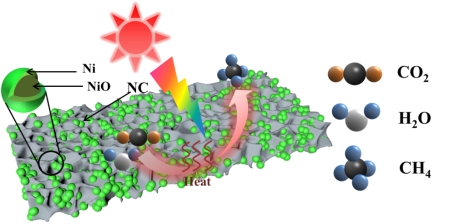
Fig 3. Schematic diagram of catalyst structure and reaction mechanism
(4) The Ru1Ni/SiO2 single atom catalyst (Fig. 4) was prepared by electrochemical replacement method. The electron transfer between Ru1 and Ni cluster was studied. Combined with Dr. Peng Zheng's theoretical calculation, it was revealed that the Ru1Ni-SAA interface was an intrinsic active site and promoted the direct dissociation of CO2, reducing the energy barrier of *CO intermediate hydrogenation, thereby improving the reaction rate of CO2 hydrogenation to CH4. This work has been published in Small (IF = 13.3) (in press. https://doi.org/10.1002/smll.202308193).
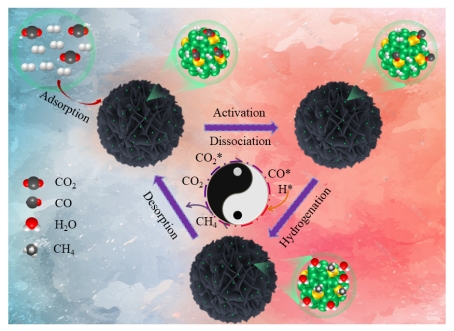
Fig 4. Schematic diagram of catalyst structure and reaction mechanism
(5) The re-dispersed NiRu/CeO2 Catalyst was prepared by high-temperature inert atmosphere treatment. The separation of metal phases in the re-dispersion process was studied. Combined with Dr. Peng Zheng's theoretical calculation, it was revealed that the newly formed Ni-Ru metal interface was conducive to the adsorption of CO2 and further direct dissociation to CO, thereby improving the catalytic performance (Fig. 5). This work has been published in Chemical Engineering Journal (IF = 13.3) (accepted).
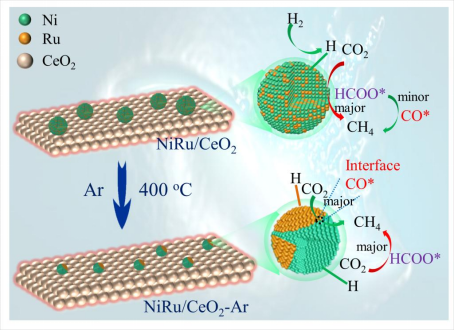
Fig 5. Schematic diagram of catalyst structure and reaction mechanism